Altered liver metabolism in offspring from diabetic mothers
04.07.2023
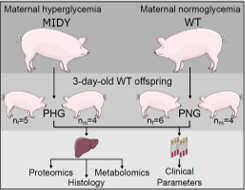
A disturbed prenatal environment is considered a risk factor for health complications in offspring. The environment of the developing fetus is influenced by an altered maternal nutritional and metabolic state. For example, in utero exposure to elevated maternal glucose can trigger long-term consequences in the physiology and metabolism of the offspring [4]. To gain mechanistic insights into adverse effects of maternal hyperglycemia on the liver of neonates, we performed a multi-omics analysis of liver tissue from piglets developed in genetically diabetic (mutant INS gene induced diabetes of youth; MIDY) or wild-type (WT) pigs. Proteome, metabolome and lipidome profiles of liver and clinical parameters of serum samples from 3-day-old WT piglets (n=9) born to MIDY mothers (PHG) were compared with those of WT piglets (n=10) born to normoglycemic mothers (PNG). Furthermore, protein-protein interaction network analysis was used to reveal highly interacting proteins that participate in the same molecular mechanisms and to relate these mechanisms with human pathology. Hepatocytes of PHG displayed pronounced lipid droplet accumulation, although the abundances of central lipogenic enzymes such as fatty acid-synthase (FASN) were decreased. Additionally, circulating triglyceride (TG) levels were reduced as a trend.
Serum levels of non-esterified free fatty acids (NEFA) were elevated in PHG, potentially stimulating hepatic gluconeogenesis. This is supported by elevated hepatic phosphoenolpyruvate carboxykinase (PCK1) and circulating alanine transaminase (ALT) levels. Even though targeted metabolomics showed strongly elevated phosphatidylcholine (PC) levels, the abundances of multiple key enzymes involved in major PC synthesis pathways – most prominently those from the Kennedy pathway – were paradoxically reduced in PHG liver. Conversely, enzymes involved in PC excretion and breakdown such as PC-specific translocase ATP-binding cassette 4 (ABCB4) and phospholipase A2 were increased in abundance. Our study indicates that maternal hyperglycemia without confounding obesity induces profound molecular changes in the liver of neonatal offspring. In particular, we found evidence for stimulated gluconeogenesis and hepatic lipid accumulation independent of de novo lipogenesis. Reduced levels of PC biosynthesis enzymes and increased levels of proteins involved in PC translocation or breakdown may represent counter-regulatory mechanisms to maternally elevated PC levels. Our comprehensive multi-omics dataset provides a valuable resource for future meta-analysis studies focusing on liver metabolism in newborns from diabetic mothers.
doi: 10.1016/j.molmet.2023.101768.